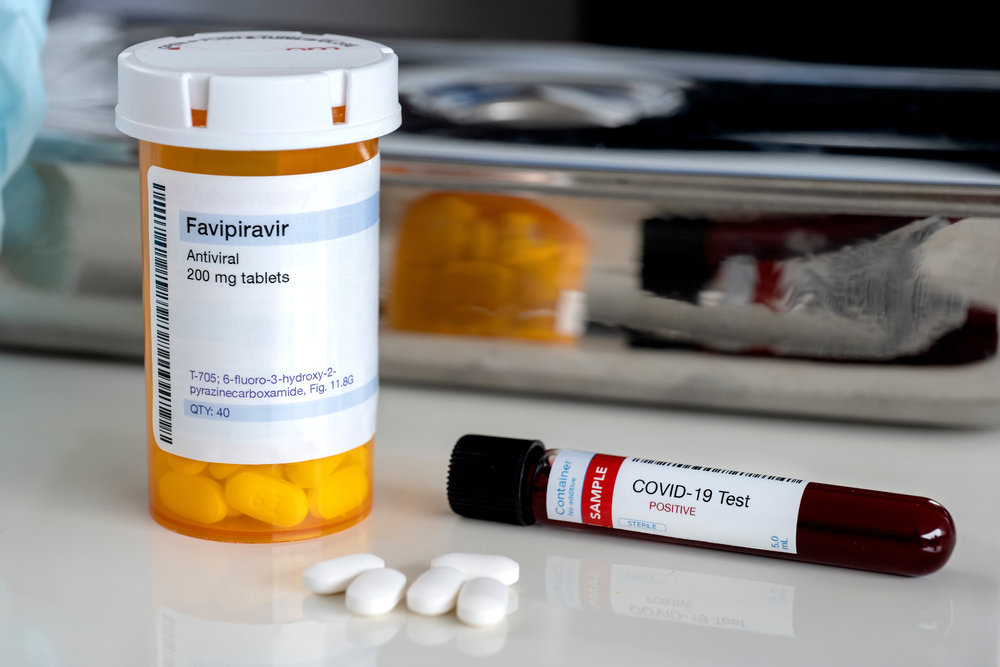
India’s drug regulatory agency on Friday approved the emergency use of a second anti-viral drug called favipiravir for treatment of mild and moderate coronavirus disease after reviewing clinical trial data submitted by a Mumbai-based company.
Glenmark Pharmaceuticals announced that the Central Drugs Standard Control Organisation (CDSCO) had approved its application to manufacture and market favipiravir for “restricted emergency use” only to meet the “unmet medical need” arising from the Covid-19 epidemic.
Under the restricted emergency use, patients will need to sign consent forms before receiving the drug.
Favipiravir is an oral anti-viral drug developed by a Japanese company and approved in Japan for the treatment of influenza. The drug has been approved for similar restricted use on Covid-19 patients in China, Italy and Russia, Glenmark officials said.
The CDSCO approval follows an application from Glenmark outlining the “interim results” of a clinical trial conducted in 10 government and private hospitals across the country involving around 150 Covid-19 patients, they said.
The results suggest a short course of treatment with the drug can lower the risk of patients with mild and moderate Covid-19 from advancing into severe disease, the officials said. The trial also suggested that the drug could reduce viral load within four to five days.
India’s drug regulatory agency has also approved remdesivir for similar restricted emergency use in mild or moderate Covid-19 patients. Four pharmaceutical companies in India will produce remdesivir under licence from the US company Gilead Sciences.“We’re producing favipiravir entirely indigenously through the local procurement of its active pharmaceutical ingredients,” a Glenmark spokesperson said. “This is also the first anti-viral approved for Covid-19 on the basis of domestic clinical trials.”
Company officials cautioned that favipiravir would be used on the discretion of medical practitioners who would determine when and which patients would qualify to receive the drug.
Doctors treating Covid-19 patients in the country also caution that clinical trials involving larger numbers of volunteers are underway in several countries and their results are still awaited.
Given that over 80 per cent of Covid-19 patients have mild illness that often improves through supportive therapy, “caution is required in interpreting the efficacy of favipiravir based on data from studies where patients receive only favipiravir”, Japanese researchers had said last month after an observational study on 2,100 patients.
Glenmark said it would provide more details from the interim results on Saturday. Its application to CDSCO for restricted emergency use of favipiravir cited these interim results along with several observations from global studies, a senior official said.A document outlining the clinical trial names the All India Institute of Medical Sciences, Raipur, Breach Candy Hospital, Mumbai, and Max Superspeciality Hospital, New Delhi, among other hospitals, as sites for the trial.