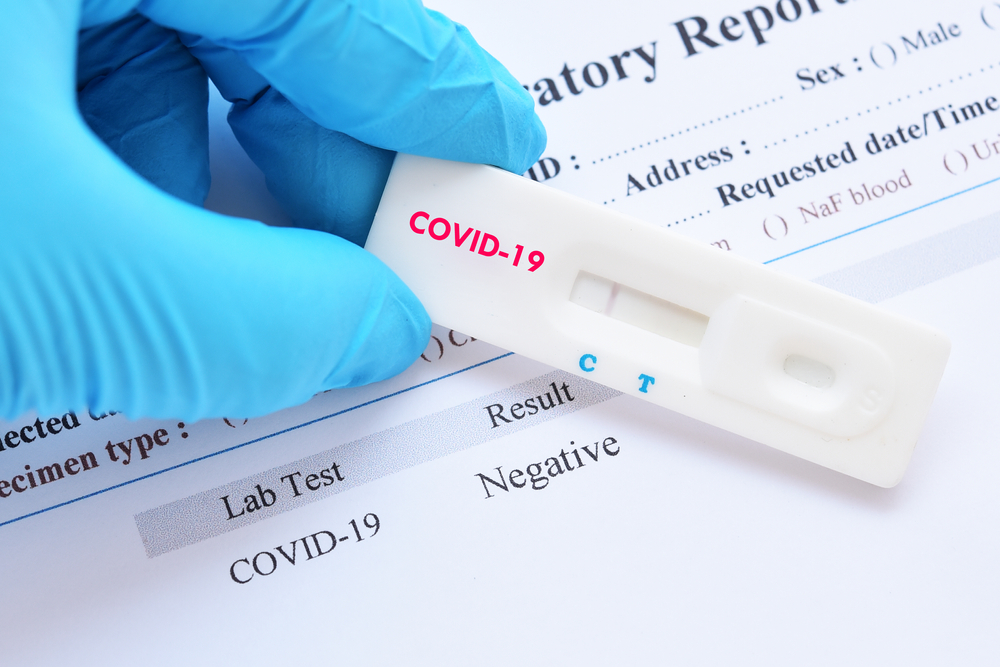
The US Food and Drug Administration on Saturday granted emergency clearance for a coronavirus testing kit that will enable individuals to take a nasal sample at home and send it to a laboratory for diagnostic testing, the second such approval it has made.
Dr Jeffrey Shuren, director of the agency’s Centre for Devices and Radiological Health, said in a statement that the new test “not only provides increased patient access to tests, but also protects others from potential exposure”. Healthcare workers can risk infection when they administer diagnostic tests.
The kit, made by Everlywell, will contain a swab for individuals to use to take a sample from inside the nostrils, and a tube filled with a saline solution to put it in for sending to one of two private lab companies: Fulgent Therapeutics or Assurance Scientific Laboratories. The company plans to partner with additional laboratories.
Some public health researchers have warned that at-home nasal swab tests can be less accurate than the specimen collection performed by healthcare providers, which involves inserting a long nasal swab through the nose into the back of the throat.
Christina Song, an Everlywell spokesperson, said consumers would first take an online screening survey to determine whether they meet federal guidelines for the test. The survey will be reviewed quickly by healthcare providers affiliated with PWNHealth, the company’s telemedicine partner. If a consumer qualifies for the test, one will be shipped out immediately.
“From the moment that you hit the order button, to the moment that you get the test results on your phone or device, that process is designed to take three to five days,” Song said.
The test kits will be available later this month, according to Song, and will cost $135.
In announcing its authorisation for the testing kit, the FDA said Everlywell had “leveraged” data from studies supported by the Bill and Melinda Gates Foundation and UnitedHealth Group to show that the specimens would stay stable during shipping.
Everlywell makes a variety of products that individuals can buy online or in stores, among them at-home test kits for diabetes, sexually transmitted diseases and high cholesterol. Some of the company’s products, such as those purporting to test for food sensitivities, have come under criticism.
New York Times News Service