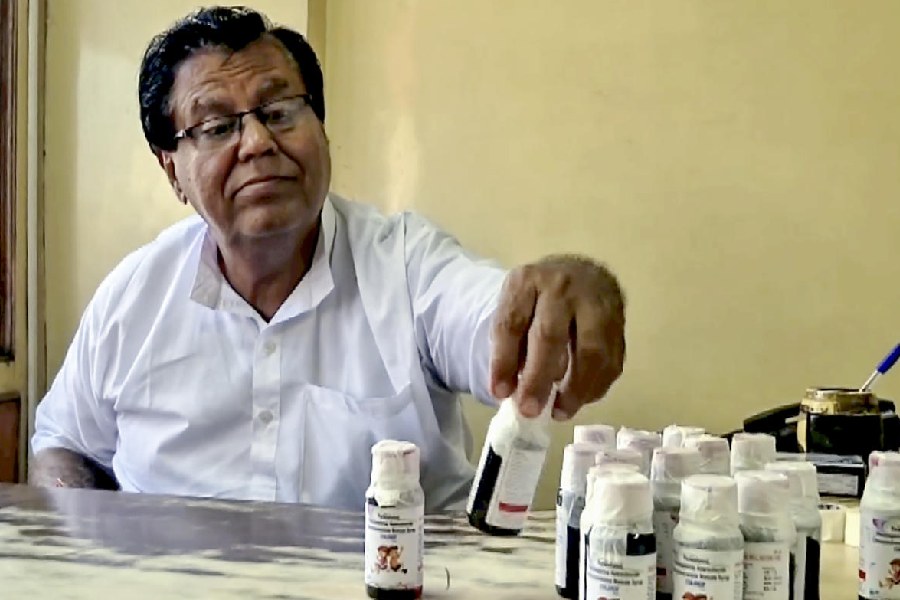
Fourteen children have died in Madhya Pradesh after consuming Coldrif cough syrup, prompting multiple state governments to issue bans on the medicine and launch investigations into its manufacture and distribution.
The Telangana Drugs Control Administration on Saturday issued a “Public Alert – Stop Use Notice” for Coldrif syrup (Batch No. SR-13), citing reports of child deaths in Madhya Pradesh and Rajasthan.
The release stated the batch had allegedly been adulterated with Diethylene Glycol (DEG), a toxic chemical.
“Stop using Coldrif syrup, Batch No. SR-13, if in possession, and report it to the local Drugs Control Authorities without delay,” the statement read.
The public may also report possession of the drug directly to the Drugs Control Administration through its toll free number on all working days.
The Madhya Pradesh government confirmed that samples of the syrup tested at the Drug Testing Laboratory in Chennai were “Not of Standard Quality” and contained 48.6% Diethylene Glycol.
The deaths occurred primarily in Parasia subdivision of Chhindwara district, with victims including children aged between 2 and 11 years.
Sub-divisional magistrate Saurabh Kumar Yadav said, “Six children are undergoing treatment, five in Nagpur and one in Chhindwara. The condition of three children admitted in Nagpur is critical.”
Chief minister Mohan Yadav called the deaths “extremely tragic” and ordered a statewide ban on Coldrif and other products from the same manufacturer, Sresan Pharmaceuticals. “Strict action will be taken against those responsible,” he stated.
Tamil Nadu health minister Ma Subramanian confirmed that the state’s Drug Controller department has been directed to conduct a detailed investigation, examining the manufacturing process, distribution, and expiry status of the medicines.
“Based on the investigation, action will be taken,” he said.
The central health ministry has also initiated inspections of 19 pharmaceutical units across six states.
Teams comprising experts from the National Institute of Virology, Indian Council of Medical Research, and AIIMS-Nagpur are analyzing samples to determine the cause of deaths.
Earlier, the Tamil Nadu government had banned the sale of Coldrif and ordered the removal of stocks from the market. According to officials, the syrup was distributed to multiple states, including Madhya Pradesh, Rajasthan, and Puducherry.
The incident has raised concerns over drug safety and monitoring of pharmaceutical companies, especially following repeated cases of DEG contamination in syrups that have resulted in child fatalities in India and abroad.