Bombay High Court on Wednesday directed the Centre to respond to a petition seeking legal steps to allow generic production of two patented anti-tuberculosis drugs currently unavailable to all patients in India who need them.
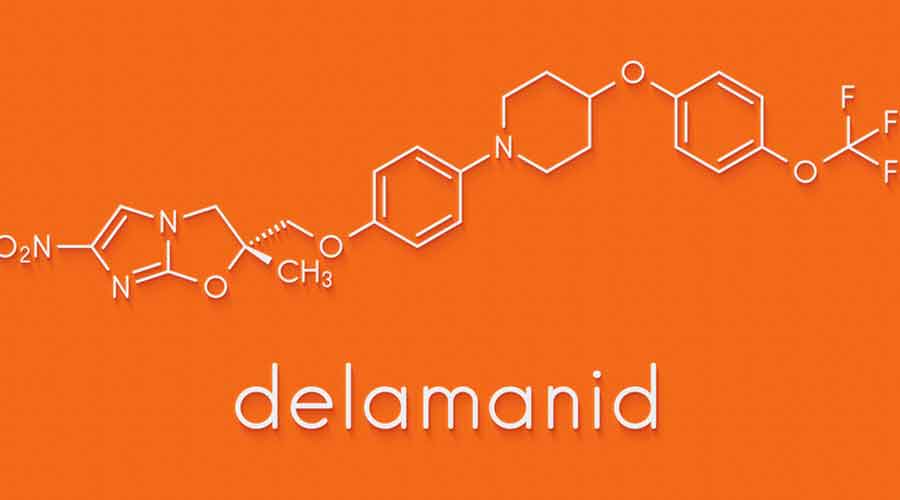
The court has asked the Centre to respond by April 28 to the petition by two TB survivors seeking a government use authorisation order or compulsory licence — provisions in Indian patent laws to allow local production of the drugs bedaquiline and delamanid.
The petitioners have said the Centre is currently relying on donations from the patent holders — the US-based Janssen Pharmaceuticals for bedaquiline and Japan’s Otsuka Pharmaceuticals for delamanid — to procure a “miniscule quantity” of the drugs compared to what India needs.
Bedaquiline and delamanid are prescribed to a subset of patients with multi-drug resistant (MDR) or extensive-drug resistant (XDR) TB that carry high mortality rates without the appropriate treatment.
The petitioners told the court that in 2019 alone, 44,724 MDR-TB patients eligible to receive bedaquiline did not get the drug and 830 patients with XDR-TB did not receive delamanid.
India’s TB control programme, in line with World Health Organisation guidelines, has recommended the use of bedaquiline and delamanid but, the petitioners have said, the limited supplies make them unavailable to patients.
Many MDR-TB and XDR-TB patients in India thus continue to receive earlier generation anti-TB drugs that carry high toxicity and have poor cure rates. The WHO has estimated that treatment success rates with those earlier generation drugs are only 57 per cent for MDR-TB and 39 per cent for XDR-TB.
“India is relying on donations for these two drugs,” said Anand Grover, director of the Lawyers Collective, a legal organisation representing the petitioners. “Without access to bedaquiline and delamanid, eligible patients face the risk of extremely high death rates.”
The petitioners, Meera Yadav and Brinelle D’Souza, both TB survivors and representing Jan Swasthya Abhiyan, a network of health rights advocates, told the court that Janssen and Otsuka are selling the drugs at high prices and government efforts to procure the drugs through tenders “have failed”.
Bedaquiline is priced at Rs 26,600 for a six-month course, and a prescribed 18-month regimen for MDR-TB patients would cost Rs 79,800 for one patient, the petitioners said, and delamanid is priced at about Rs 91,000 for a six-month course.
However, a team of UK researchers have estimated that generic production could slash the price of both drugs to below Rs 10,000 per patient for a six-month course.
Several manufacturers in India have said they have the capacity and technology to make generic versions of the drugs but no licensing pacts have emerged yet, the petitioners have pointed out.
Health activists say the Centre’s inaction on taking legal steps to facilitate generic production of bedaquiline and delamanid contrasts with the Indian government’s application at the World Trade Organisation seeking waiver of intellectual property rights on Covid-19-related technologies.
“For Covid-19, India proposed waiver of all patents on medical products. It is time drug resistant TB which is a public health emergency received similar attention,” Leena Menghaney, a lawyer and an adviser with the global humanitarian agency Medicins Sans Frontieres, told The Telegraph.
Doctors and health activists said the Covid-19 mortality rate was significantly smaller than the mortality rate among patients with MDR-TB and XDR-TB. “It is time this appeal from TB survivors is considered by the government to encourage generic drug makers to enter the supply chain,” Menghaney said.