New Delhi, March 21: Ranbaxy Laboratories Ltd today said it has purchased patents, trademarks and automated manufacturing equipment from Senetek Plc’s proprietary disposable auto injector for an undisclosed sum.
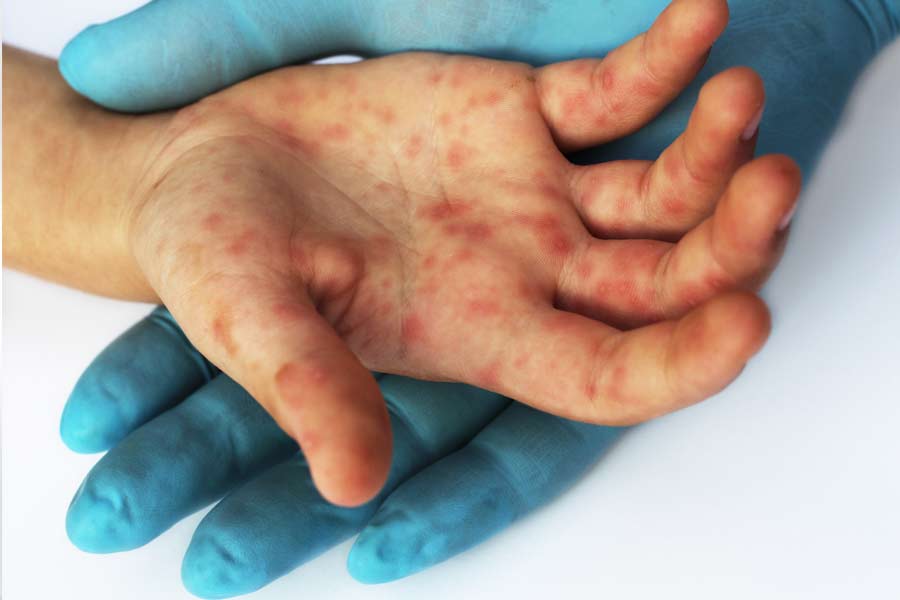
The company said the acquisition is for self-administration of parenteral drugs, including epinephrine (also known as adrenaline), for emergency treatment of severe allergic reaction from peanuts and other allergies.
Parenteral is a term used to describe drugs that are injected beneath the skin ? intravenous or intramuscular.
The deal was executed by Ranbaxy’s wholly-owned US subsidiary, Ranbaxy Pharmaceuticals Inc.
Initially, Ranbaxy will focus on pre-filling the auto-injector device with epinephrine. It will also evaluate the development of other parenteral drugs, including Senetek’s patented medicine for erectile dysfunction, Invicorp.
The agreement entails a non-refundable payment by Ranbaxy to Senetek on signing and milestone payments based on regulatory approvals and cumulative sales milestones.
The terms also include a percentage of Ranbaxy’s and its licensees’ quarterly net sales of the products to be paid to Senetek.
Ranbaxy will make infrastructure investments, including building the clean-room suites at its facility in New Jersey, to house the highly automated auto-injector production line.
Ranbaxy will obtain regulatory approvals and market the product. It has also agreed to discuss the possibility of manufacturing and supplying Invicorp in the auto-injector device to Senetek’s licensees.
“Anaphylactic shock due to allergic reaction to peanut-based food additives is a growing health risk that results in over 30,000 emergency cases and 150 to 200 preventable deaths in the United States every year. This excludes an additional 50 deaths from bee stings and other allergic reactions,” said Dipak Chattaraj, chairman of Ranbaxy Inc.
Chattaraj said Senetek’s patented modular autoinjector “is ideally suited for this acute self-medication market, which is growing at over 25 per cent annually, as health and educational organisations generate awareness of these risks”.
The device can also be adapted for other acute applications, including the military and homeland security sectors for administration of antidotes to chemical and biological agents.