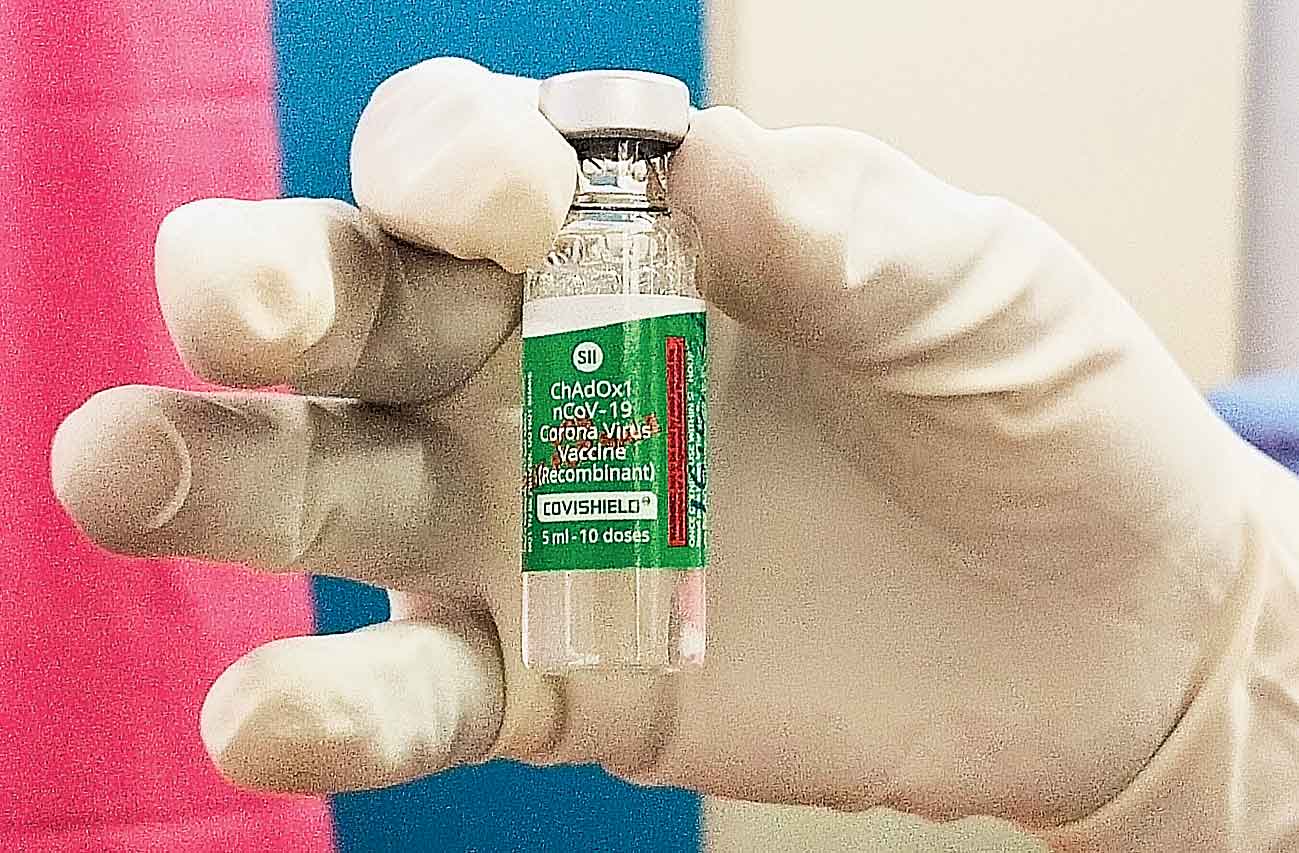
India's drug regulator DCGI has extended the shelf life of Covishield, the Oxford-AstraZeneca COVID-19 vaccine, from six to nine months from its manufacturing date.
In a letter to the Serum Institute of India, Drugs Controller General of India V G Somani said the SII is permitted to apply the shelf life of nine months to unlabelled vials available on hand.
Shelf life is the length of time for which an item remains fit for use.
The DCGI said it has no objection in respect of 'Extension of Shelf Life of Covishield Vaccine' in multi-dose glass vial (10 dose-5ml) from six months to nine months.
"You are permitted to apply the shelf life of nine months to unlabelled vials available on hand, subject to the condition that the details of such stock, batch-wise, shall be submitted to this office and Central Drugs Laboratory, Kasauli," Somani said in the letter.