Mumbai: Sun Pharmaceutical Industries has received its first drug approval in more than four years for its Halol plant from the US Food and Drug Administration (US FDA).
Last month, the US drug regulator had given its clearance, which meant that Sun Pharma could resume supplies to the US market from Halol in Gujarat.
In a regulatory filing with the bourses on Wednesday, Sun Pharma said it had obtained the nod from the US FDA for its Infugem injection used for the treatment of cancer.
The addressable market size was approximately $35 million for the year ended March 2018, Sun Pharma said.
The company had received an establishment inspection report (EIR) from the US FDA for the Halol facility in Gujarat. This came after an inspection by the regulator between February 12 and 23.
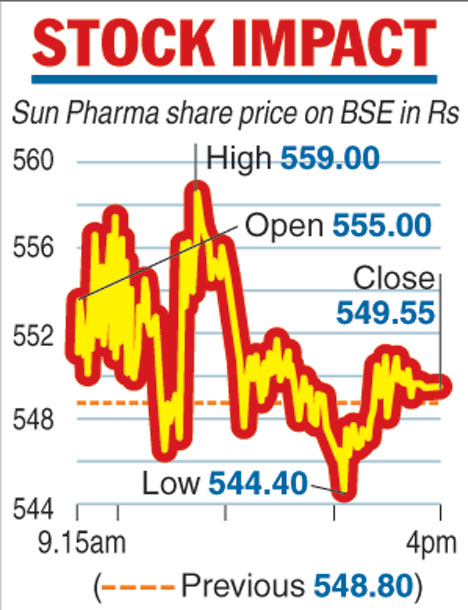
Shares of the country's largest pharmaceutical firm ended marginally higher on the news. On the BSE, the Sun Pharma scrip settled with gains of 0.14 per cent at Rs 549.55.
The US drug regulator had first issued Form 483 with more than 20 observations on the plant in 2014. This was followed by a warning letter over the violation of manufacturing norms in December 2015.
Sun Pharma was unable to export new drugs from the facility as a result of the warning.