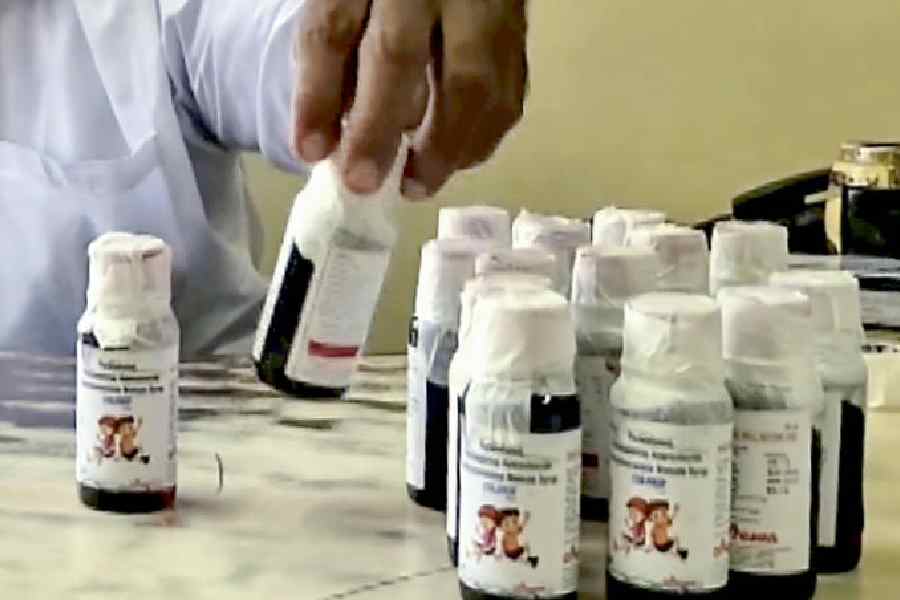
A doctor in Madhya Pradesh’s Chhindwara district has been arrested following the deaths of 14 children allegedly linked to contaminated Coldrif cough syrup, NDTV reported quoting officials.
Most of the affected children had been treated at the private clinic of Dr Praveen Soni, a government-appointed doctor practising in Parasia, according to media reports.
Soni is accused of prescribing Coldrif syrup to children who presented with routine cold and fever symptoms in early September, according to a report in The Times of India.
Following initial recovery, the children’s conditions worsened, with reduced urine output and kidney infections, ultimately leading to fatalities. Kidney biopsies later revealed diethylene glycol contamination in the syrups.
The Madhya Pradesh government has banned the sale of cough syrup due to suspected renal failure, with officials stating the drug samples were found to contain a highly toxic substance.
The Central Drugs Standard Control Organisation (CDSCO) has also initiated risk-based inspections at the manufacturing units of 19 drugs, including cough syrups and antibiotics, across six states, the Union health ministry said on Saturday.
A sample of the syrup, tested by the government drug analyst at the Drug Testing Laboratory in Chennai, was declared "Not of Standard Quality" by the Tamil Nadu Directorate of Drug Control, officials said here.
The action by the Madhya Pradesh government came in the wake of the deaths of 14 children in Chhindwara district due to suspected kidney failure. Of these, 10 deaths were reported in Parasia subdivision since September 7, local officials said.
Financial assistance of Rs 4 lakh was sanctioned for the kin of the 14 deceased children, officials said.
Of those who died, 11 were from Parasia sub division, two from Chhindwara city and one from Chaurai tehsil.
SDM Yadav said that as a precautionary measure, the local administration had already banned the sale of Coldrif and another cough syrup, 'Nextro-DS', on Monday. The test report for Coldrif arrived on Saturday, while that of Nextro-DS was awaited.
The Tamil Nadu drug control authorities, in their report dated October 2, declared the Coldrif syrup sample (Batch No SR-13; Mfg: May 2025; Exp: April 2027) manufactured by Sresan Pharmaceuticals, Kancheepuram, as adulterated because it contained diethylene glycol (48.6% w/v), a poisonous substance "which may render the contents injurious to health".
Following the report, the Madhya Pradesh Food and Drug Administration issued instructions to stop further sale and distribution of Coldrif statewide and immediately seize any available stock for investigation under the Drugs and Cosmetics Act, 1940. It also ordered that other products manufactured by Sresan Pharmaceuticals be removed from sale pending testing.
Chief Minister Mohan Yadav, in a post on X, called the deaths "extremely tragic" and said strict action will be taken against those responsible.
"The sale of this syrup has been banned across Madhya Pradesh. A ban is also being imposed on other products of the company that manufactures the syrup," the CM stated.
The Tamil Nadu government on Friday banned Coldrif following reports of deaths in Madhya Pradesh and at least three similar fatalities in Rajasthan due to suspected kidney infections.
Samples from the affected children have been sent to the National Institute of Virology in Pune, while further tests on the syrup's adulteration and contamination are underway.
The CDSCO has initiated risk-based inspections at the manufacturing units of 19 drugs, including cough syrups and antibiotics, across six states, the Union health ministry said.
A team comprising experts from the National Institute of Virology, the Indian Council of Medical Research, National Environmental Engineering Research Institute, CDSCO and AIIMS-Nagpur, among others, are analysing various samples to assess the cause of deaths in and around Chhindwara, it stated.
India has faced scrutiny over the quality of its pharmaceutical exports after the World Health Organization linked cough syrups made by another company to the deaths of 70 children in Gambia in 2022 — a finding that New Delhi later disputed.