India’s Covid-19 vaccine shortages may persist for three more months until production capacity is ramped up and new vaccines arrive, the Union health ministry signalled on Thursday with a top health official urging patience.
The health ministry has for the first time released vaccine availability data up to July 2021, showing that India has an assured quantum of 516 million doses, enough to vaccinate only about 250 million of the country’s estimated 900 million adults.
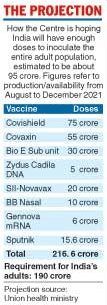
Increased production of Covishield and Covaxin, the introduction of Russia’s Sputnik V, and five new vaccines will give India access to over two billion doses between August and December this year, said Vinod Paul, chair of the national expert group on Covid-19 vaccine policy.
“Be proud to say that two billion doses in a matter of five months will be made in India for the people of India,” Paul said. “Vaccines will be available for all as we move forward…. If I extend this estimate to the early first quarter (of 2022), this number is three billion.”
“After this data, there should be no doubt. All will get vaccines,” added Paul, who is a senior paediatrician and a member of Niti Aayog, India's apex government think tank.
The assurances come at a time states and private hospitals across the country have reported shortages of vaccines as they seek to inoculate adults aged between 18 and 45, eligible under the revised vaccination policy, while the Centre continues to vaccinate all adults 45 years or older.
Paul, providing insights into the health ministry’s expectations, said India would have 750 million doses of Covishield, 550 million doses of Covaxin, 156 million doses of Sputnik V and over 700 million doses of new vaccines from Bio-E, Bharat Biotech, Serum Institute, Zydus Cadila and Gennova.
India’s drug regulatory authority has already approved Sputnik V, which is expected to enter the vaccination campaign within days. But the five other vaccines are in various stages of clinical development and would need to prove their efficacy before they are approved.
Senior researchers familiar with vaccine science said the prospect of over two billion doses within five months was optimistic and would hinge on all five vaccines being approved and produced.
“It would depend on clinical trial results — this is achievable in a best-case scenario,” said a senior virologist. Another vaccine researcher said the expectation of five new vaccines within three months was “very optimistic”.
Multiple health experts have criticised what they view as a failure of India’s vaccine policy-makers to anticipate and prepare for the doses required through advance investments and purchase pacts with vaccine makers between July and December 2020 as many countries have done.
India had till Thursday administered 180 million doses, but only about 10 per cent of the population has been covered by at least one dose, compared with 46 per cent of the US population and 15 per cent of Brazil’s population.
Paul said an asymmetry between supply and demand had been seen across the world at the start of vaccination campaigns. “We are moving through a phase where there is finite supply…. It takes time. It takes time to move out of this phase,” he said.
“In this phase, whatever supply is available, every dose should reach everyone with patience,” Paul said. “To cover the entire nation, please remember, it will take a little while and we should accept this as a reality, with humility, giving credit to scientists, industry, health workers, doctors and district teams for the efforts they have taken.”
Health experts have criticised what some view as an abrupt policy change by the Centre to allow the states and private hospitals the flexibility to inoculate all adults 18 years or older from May 1 without making provisions for the extra doses needed.
But Paul defended the policy change, saying the states and the people of India had sought it.
He said the Centre’s earlier policy to inoculate adults 45 years or older was based on the need to protect the most vulnerable people first. “But states wanted flexibility to take decisions. In a federal system, the Centre agreed. People wanted it, the states wanted it. We must now work towards the seamless delivery of vaccination.”
Foreign jabs
The Covid-19 vaccines from Pfizer, Moderna and Johnson and Johnson are unlikely to be available in India until at least the third quarter of 2021, Paul said, providing insights into the outcomes of talks the Indian government has had with the three companies.
“We’ve been in touch with Pfizer, Moderna and J&J from the start,” Paul said. “We’ve asked them, ‘Please would you like to send doses to India, please would you like to manufacture in India? We will find partners, we’ll assist you’.”
Paul said the companies had indicated they would consider India in the third quarter of the year. “We’re talking with all three companies now. This is being pursued at the highest diplomatic level. We’re inviting (them) to make (the vaccines) here, and to transfer the technology to India.”
Health experts tracking global vaccine supplies have said the companies are unlikely to send doses to India for weeks because of the advance purchase orders they have received from other countries such as Australia, Brazil, Canada, Japan, Britain, the US and the European Union.