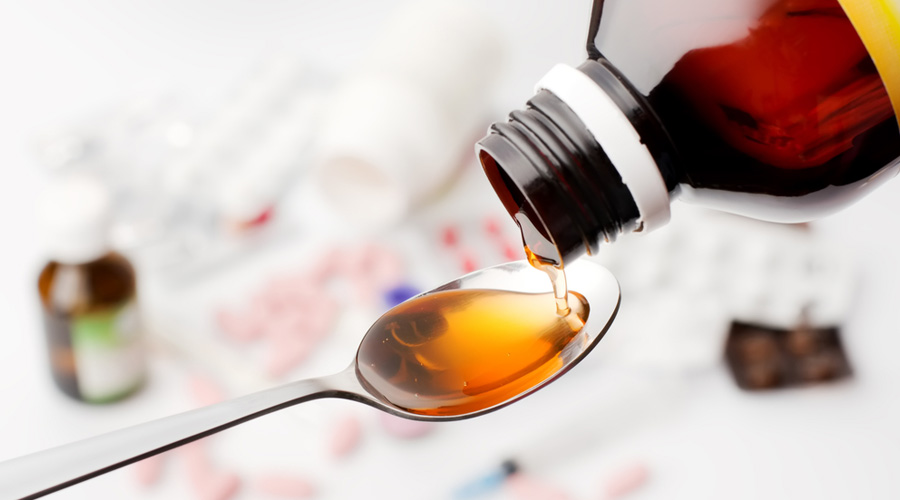
The World Health Organisation on Monday released what it said was “an urgent call” to countries to prevent, detect and respond to incidents of substandard and falsified medical products, citing contaminated Indian-made syrups linked to child deaths in the Gambia and Uzbekistan.
The global health agency has called on regulators and governments to assign resources to improve risk-based inspections of manufacturing sites, increase market surveillance, including risk-based testing, and enforce measures to help combat the manufacture, distribution and use of substandard and falsified medicines.
The WHO has since October issued three global alerts cautioning against cough syrups contaminated with toxic chemicals called diethylene glycol and ethylene glycol — mentioning four products from Maiden Pharmaceuticals and two from Marion Biotech, both private Indian firms, and four products from three Indonesian firms.
“The cases are from seven countries, associated with more than 300 fatalities in three of these countries. Most are young children under the age of five,” the WHO said in Monday’s statement without specifying the country-wise breakup of fatalities.
However, health authorities in the Gambia had in early October linked the deaths of at least 66 children to cough syrups from Maiden Pharmaceuticals, while Uzbekistan’s health ministry had linked at least 18 child deaths to a cough syrup from Marion Biotech.
India’s drug regulatory authorities initiated probes into both Maiden’s and Marion’s production facilities and ordered Maiden’s shutdown after drug inspectors detected flaws in its manufacturing standards. But drug regulators have remained silent about their probe into Marion.
Drug regulators have said the cough syrups from Maiden flagged by the Gambia and those from Marion flagged by Uzbekistan were intended for export and not for sale in the Indian market. Queries sent by this newspaper to the Union health ministry about the outcome of its probe into Marion’s facilities have not evoked any response.
Even after detecting the manufacturing flaws at Maiden’s production facilities, India’s chief drug regulator V. Somani had in December written to the WHO questioning the absence of evidence that the cough syrups had caused the deaths of children in the Gambia.
The WHO has asserted that the contaminants are toxic and used as industrial solvents and antifreeze agents that can be fatal even if taken in small amounts and “should never be found in medicines”.
Independent medical experts, including an Indian paediatrician who has earlier investigated ethylene glycol contamination, say a cause-effect link is difficult to establish in these circumstances and that the mere detection of such toxic chemicals in medicines should be a cause for alarm and action.