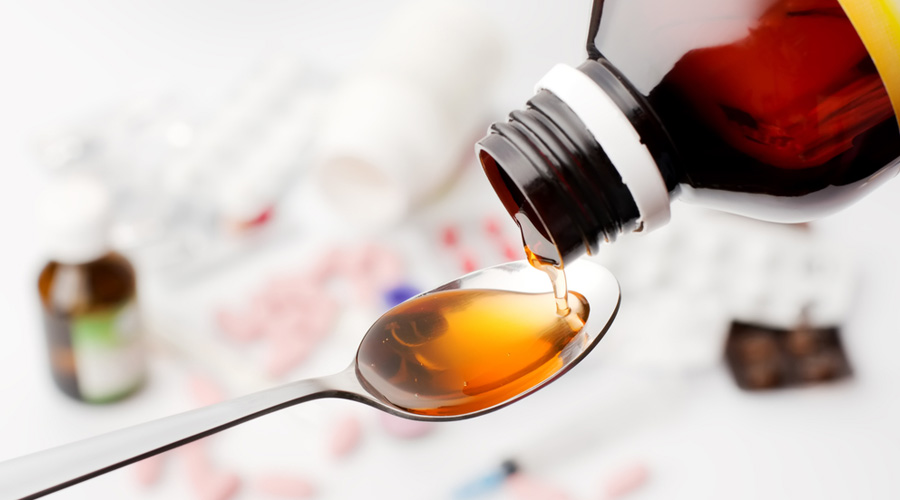
A cough syrup produced in Punjab, marketed by a Haryana firm and sold in distant islands in the Pacific had toxic contaminants, the World Health Organisation said on Tuesday, flagging a fresh instance of substandard drugs from India.
The WHO said that samples of the syrup from a batch in the Marshall Islands and Micronesia were found to contain “unacceptable amounts of diethylene glycol and ethylene glycol”, chemicals toxic to humans that can prove fatal when consumed.
The syrup’s stated manufacturer is QP PharmaChem Ltd in Punjab and its stated marketer is Trillium Pharma in Haryana, the WHO said in a medical alert. “To date, neither the stated manufacturer nor the marketer has provided guarantees to WHO on the safety and quality of these products,” it said.
The product cited in the alert may have marketing authorisations in other countries in the western Pacific region and may have also been distributed through informal markets to other countries or regions, the WHO has said.
Samples of the syrup, which contains a compound called guaifenesin — an expectorant used to relieve chest congestion and cough — from the Marshall Islands were analysed by quality control laboratories of the Therapeutics Goods Administration, Australia’s drug regulatory authority.
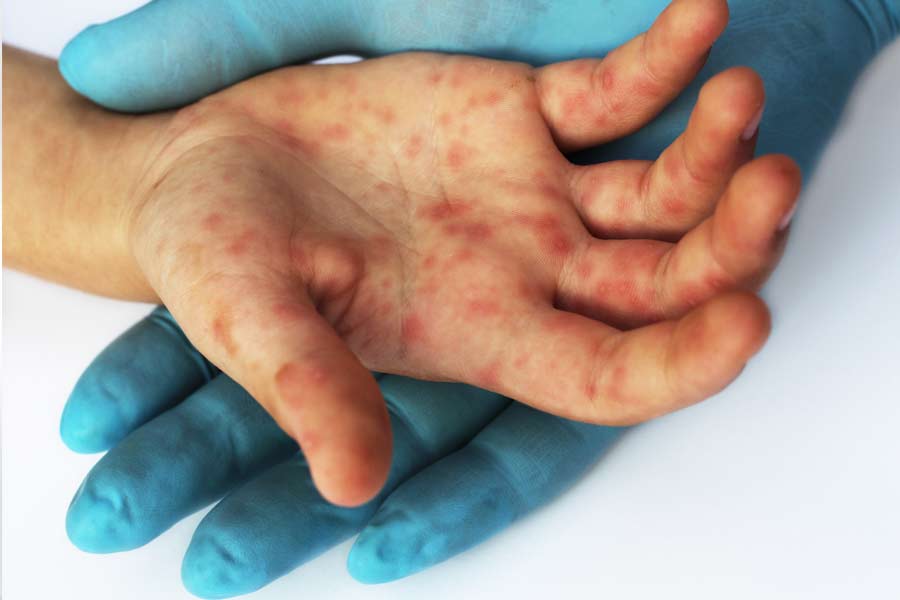
The TGA analysis found the product to contain the two contaminants. The WHO said the product is unsafe and its use, especially in children, may result in serious injury or death. The toxic effects can include abdominal pain, vomiting, diarrhoea, headache, and acute kidney injury that may lead to death.
A PharmaChem executive contacted by this newspaper said the company had exported the cough syrup to Cambodia three years ago and never to the Marshall Islands or Micronesia. “We exported 18,336 bottles to Cambodia in 2020. The product would have expired by now,” said the executive Sudhir Pathak.
“We do not export to Marshall Islands or Micronesia — we have no idea how the product was available there. This needs to be investigated. We don’t understand why they tested samples which have expired,” Pathak said over telephone.
There was no response on a phone number listed for Trillium Pharma.
A query from this newspaper to the Union health ministry on whether India’s drug regulatory authorities have had knowledge or oversight over the production and export of the syrup to the Marshall Islands and whether it is also sold in India has evoked no response yet.
A QP Pharmachem executive, reached on the telephone by this newspaper on Tuesday, said he would respond later on Tuesday night.
Tuesday’s WHO alert is the fourth instance since October of foreign health authorities raising quality concerns about drugs from India. Authorities in the Gambia and Uzbekistan had linked child deaths last year to Indian-made cough syrups contaminated with the same compounds found in the syrup from the Marshall Islands.