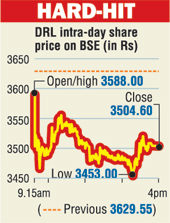
Mumbai, Nov. 9: Drug giants Sun Pharma and Dr Reddy's Laboratories got floored on the bourses today, punished for poor results in case of Sun and US concerns over manufacturing in case of the latter.
Amid massive volatility in the wake of the Bihar poll results, Dr Reddy's lost 3.44 per cent, or Rs 124.95, to finish at Rs 3,504.60; Sun Pharma fared worse - a loss of 6 per cent, or Rs 46.80, to end at Rs 756.90.
At a conference call with analysts today, Dr Reddy's said that the warning letter given by the US Food and Drug Administration (FDA) to three of its facilities may affect approval of new applications from these sites. Simultaneously, the company is looking at the possibility of transferring certain products to alternate locations.
"Till the matter is resolved, the agency (FDA) may withold approval of new applications which involve these sites," G.V. Prasad, chief executive officer, Dr Reddy's told the analysts. He added that the regulator had cited violations relating to documentation, practices and controls apart from laboratory testing procedures and incident investigating practices .

Prasad said that presently there was no directive from the FDA to stop manufacturing activity or shipment of any products from these facilities.
"We have instituted corrective action plan to address the (Form) 483 observations received earlier in each of these sites which formed part of the updates shared with agency," Prasad added.
52-week low
Shares of Sun Sun Pharma today hit a 52-week low at the stock exchanges on disappointing second quarter results and apprehensions with regard to its Halol facility.
Sun Pharma is now engaged in the process of addressing deviations in current good manufacturing practices at this facility and there are reports that the plant has got an OAI (Official Action Indicated) tag from the FDA, raising fears of a warning letter from the FDA.