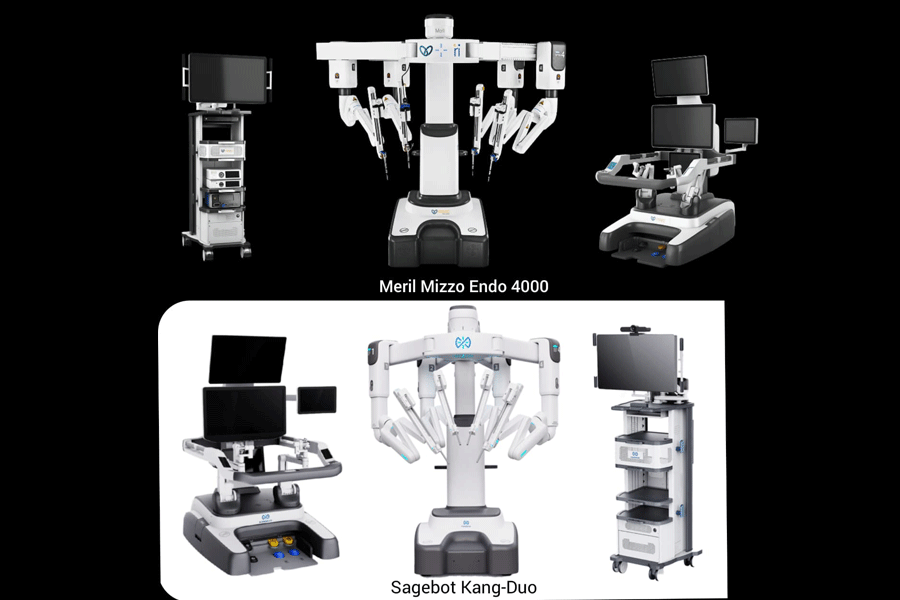
A dispute has erupted over the origins of a surgical robot launched in India this month, with one company claiming it is a rebadged Chinese system and its maker asserting that it is an India-manufactured product.
Sudhir Srivastava Innovations (SSI), a Gurgaon-based robot-maker, has written to health authorities alleging that Meril — a firm based in Vapi, Gujarat — has imported a fully manufactured Chinese-made surgical robot, KangDuo SR2000, and misrepresented it as its own product, Mizzo Endo 4000.
Meril has rejected the allegation, saying its Mizzo Endo 4000 system was assembled and validated locally under a government licence. It says the core assembly, software integration and quality checks were performed entirely in India and only select components were sourced from abroad.
The competing claims, some industry executives say, underscore broader questions about regulatory oversight and the scope for companies to blur the lines between imported and homegrown products under the Narendra Modi government’s Make-in-India initiative.
Meril has described Mizzo Endo 4000 as a “future-ready platform”.
Mizzo Endo 4000 is designed to support a wide range of procedures, covering specialities from colorectal, gastrointestinal and gynaecological operations to ENT and oncology surgeries.
Surgical robots are surgeon-operated tools that enhance precision and dexterity during complex procedures, often allowing smaller incisions and faster patient recovery.
SSI has asked the Central Drugs Standards Control Organisation (CDSCO), India’s apex regulatory authority for medicines and medical devices, to investigate Meril for alleged misrepresentation of a Chinese robot.
In a letter to the CDSCO on Wednesday, SSI cited import records that it said showed that Meril had brought in fully manufactured surgical robots, named KangDuo 2000, from China and relabelled and repackaged them as Mizzo Endo 4000 “without any local assembly or value addition”.
“Such actions dilute the credibility of genuinely indigenous companies that have invested years of efforts and significant resources in innovation, validation, and regulatory compliance,” the letter said.
It also said that any misrepresentation of imported devices as locally made “violates the intent” of the Make-in-India initiative, launched in September 2014 to promote innovation, investment and manufacturing in India.
Meril’s chief executive officer, Vivek Shah, has asserted that Mizzo Endo 4000 is manufactured in India under a valid CDSCO licence.
“Mizzo Endo 4000 is an India-developed product, fully integrated and validated at our own facility in compliance with Indian regulations,” Shah told The Telegraph.
“While select high-quality components are sourced globally — as is (the) standard in advanced robotics — the safety architecture, core assembly, software integration, and quality checks are entirely performed in India,” Shah said.
“Leveraging a global supply chain while building domestic capability is fully aligned to generate employment and expertise within India.”
Some industry observers and surgeons familiar with surgical robotics say the dispute reflects not only questions of provenance but also the high stakes in India’s expanding surgical robotics market, which is estimated to host nearly 500 systems nationwide.
India’s surgical robotics market is projected to grow from $851 million in 2023 to nearly $4 billion by 2031, according to a July 2025 market intelligence report, published on the website of the US International Trade Administration.
The report estimates that 70 per cent of India’s medical devices are imported. Industry executives say American robot maker Intuitive Surgicals dominates the market, though domestic companies like SSI and Meril are expanding their presence.
Cardiac surgeon Sudhir Srivastava founded SSI in 2010 to develop homegrown surgical robotics systems priced below imported systems. The company introduced its indigenous robot, Mantra, in 2022 and has since sold some 120 units.
“We spent nearly five years to develop Mantra, our engineers worked in the lab, we went through the full process of pre-clinical studies and human clinical trials ahead of the CDSCO approval,” Srivastava told this newspaper. “Portraying imported products as India-made undermines the credibility of the Make-in-India initiative.”
Meril is also competing, with locally made surgical robots and what a company spokesperson described as an “ecosystem” designed to enable deployment even in smaller towns.
“We have installed robots in Himmatnagar, Yavatmal and Belgaum,” the spokesperson said.
The market report estimates that India has more than 850 surgeons trained in robotic-assisted surgery. Over the past decade, more than 12,800 surgeries in the country have been performed using robots.