The Covid-19 pandemic sweeping the globe has yet to peak. India’s under lockdown. So what’s in the pharmaceutical pipeline to either treat, cure or stop Covid-19 dead in its tracks?
Researchers worldwide are racing to repurpose old drugs for treating the virus or come up with new ones as well as working on vaccines. Governments from the US to China are throwing support behind the efforts to overcome the pandemic, seeing it as a national security issue. Dr Nevan Krogan, a biosciences professor at the University of California, says the challenge facing researchers now is what it must have felt like to be “in the German Enigma code-breaking group in World War II.”
Scientists studied the genes of the coronavirus cdc.gov
Scientists have just published on the website bioRxiv a list of 69 drugs and experimental compounds that they believe can work against the coronavirus and which will now be tested to see if they’re effective against Covid-19. Scientists studied the genes of the coronavirus. Viruses replicate by injecting their genes into human cells which go on to produce viral proteins that then make copies of the invading virus.
As you’d expect, the scientists identified anti-viral drugs. But they also found potentially useful drugs prescribed for widely different categories of illnesses including malaria, Parkinson’s, diabetes, blood pressure, schizophrenia and cancer. Intriguingly, the list also includes antibiotics which normally believed to be of no help in attacking viruses.
In one major initiative, the World Health Organization has launched a multi-country drive called SOLIDARITY to isolate drugs approved by regulators for other diseases that can be utilised in the Covid-19 fight. The reason they’re looking at already safety-tested drugs is that these don’t need lengthy clinical trials and many are already on the market
The WHO said drugs lined up for immediate testing are antiviral intravenous drug remdesivir; HIV combination lopinavir and ritonavir sold as Kaletra; and anti-malarial chloroquine, which was among the medicines identified by bioRxiv website, and a related drug, hydroxychloroquine. So far, 10 countries -- Argentina, Bahrain, Canada, France, Iran, Norway, South Africa, Spain, Switzerland, and Thailand -- have signed up to participate in the SOLIDARITY quest.
“This trial focuses on the key priority questions,” Ana Maria Henao-Restrepo, a senior WHO official, said. “Do any of these drugs reduce mortality? Do any of these drugs reduce the time a patient’s in hospital and whether the patients receiving any of the drugs needed ventilation or intensive-care units,” she said.

Studies suggest antimalarial drugs like chloroquine and hydroxychloroquine, prevent the virus from invading cells and replicating Shutterstock
Chloroquine trials encouraging so far
A just-completed French trial of two dozen patients suggests chloroquine may speed recovery in Covid-19 patients. In the trial, only 25 per cent of patients given the medicine tested positive for the virus after six days while 90 per cent of those who didn’t get the medicine were still positive. The French government’s planning now to expand the study.
There have also been positive reports from doctors in China and South Korea who used chloroquine and hydroxychloroquine -- both of which have the advantage of being decades old, off-patent and cheap. Studies suggest the antimalarial drugs prevent the virus from invading cells and replicating. Besides shortening the illness, researchers believe the drugs might help stop people getting infected in the first place.
Separately, researchers at France’s IHU-Mediterranee Infection in the southern French city of Marseille said that administration of a combination of the antimalarial hydroxychloroquine and the antibiotic azithromycin to Covid-19 patients over a five-day period resulted in “a significant reduction” in viral load.
Another drug possibility is Gilead Sciences Inc’s experimental antiviral compound remdesivir. The US pharma giant initially developed the drug for treating Ebola but it didn’t make the cut against rival medicines. Researchers now are looking at this therapy to see whether it might interfere with Covid-19’s ability to replicate within the body.
Doctors in China reported in the scientific journal Nature they found remdesivir to be “highly effective” in controlling Covid-19 “in vitro,” meaning in a laboratory situation. Now, it’s to be seen whether it’s equally effective when used on people. Gilead has said it’s launching two Phase III clinical trials this month of remdesivir in adults diagnosed with COVID-19.
The trials will involve 1,000 patients mostly in Asia, as well as in countries that have had high numbers of diagnosed cases like Italy which is the European epicentre of the pandemic. Gilead’s chief executive Daniel O’Day said by the end of April, “we should have a preliminary idea of the safety and efficacy of this medicine against coronavirus. These trials are on top of two clinical trials in China’s Hubei province and another by the US National Institute of Allergy and Infectious Diseases trial. The China trial data is expected in April.
Another drug generating excitement is arthritis drug Kevzara. It’s being investigated as a way to alleviate the severest symptoms of Covid-19. US drugmaker Regeneron Pharmaceuticals and French pharma company Sanofi are beginning trials of the arthritis drug in the hopes of replicating positive findings reported by doctors in China treating Covid-19 patients. Regeneron and Sanofi have launched a US clinical trial of Kevzara -- pharmaceutical name sarilumab -- in 400 COVID-19 patients hospitalised for severe illness. As the virus spread in China, doctors frantically tried a number of drugs on patients to see if any helped in tackling the virus. Kevzara works by blocking inflammation which occurs in people with rheumatoid arthritis. Inflammation is the body's natural response to infection but in patients with coronavirus it can spin out of control, worsening symptoms.
Roche’s arthritis drug Actemra is also getting attention. Actemra already has got anecdotal support from Chinese doctors for use in treating life-threatening lung inflammation in patients with Covid-19 and now the Swiss drugmaker is launching clinical trials on hospitalised patients with severe Covid-19 pneumonia to seek more evidence for its effectiveness. Chinese officials said the drug was “clearly effective” in treating Covid-19.
Eli Lilly, meanwhile, has teamed up with Vancouver-based biotech AbCellera to develop antibody treatments for Covid-19. Taking a blood sample from one of the first Covid-19 survivors, AbCellera screened over five million immune cells looking for ones that made antibodies which helped the patient neutralize the virus and recover.
The next step is to screen these antibodies to find ones that work best in neutralising the virus. The companies are hoping to create a treatment ready for human trials within four months. Japanese pharma giant Takeda also is working on a plasma-derived therapy using blood from people who’ve survived Covid-19 and isolating the protective antibodies which helped the patients recover. Takeda’s hoping to come up with a drug within 12-18 months. The concept of using blood from survivors with immunity to battle an illness is more than a century old and has been used to fight Ebola and H1N1.
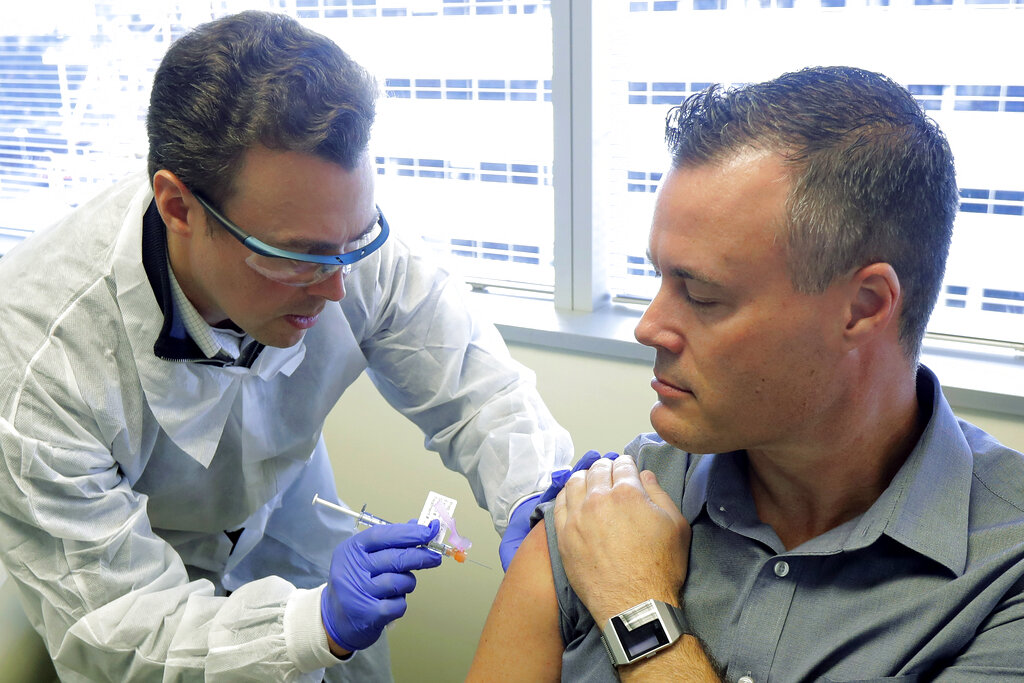
Pharmacist Michael Witte, left, gives Neal Browning a shot in the first-stage study of a potential coronavirus vaccine Monday, March 16, 2020, at the Kaiser Permanente Washington Health Research Institute in Seattle. AP
Experimental vaccines
Also, the World Health Organization says 35 experimental vaccines are in development. One firm out in front is Boston-based biotech Moderna which came up with a vaccine candidate in a record seven weeks after Chinese researchers sequenced Covid-19’s genetic material. The Chinese shared the results in January, permitting researchers globally to grow the live virus and track how it breaks into human cells and hijacks their reproductive capacity to copy itself.
Moderna’s vaccine is using a synthetic strand of messenger RNA -- otherwise known as mRNA -- that seeks to persuade the body’s cells to manufacture an immune response to an infection. The trial involving 45 young, healthy volunteers is being conducted at the Kaiser Permanente Washington Health Research Institute in Seattle which has been badly hit by an outbreak. The goal of the test is to evaluate any safety issues and determine the appropriate dose to stimulate an effective immune response.
Then, there’s China’s CanSino Biologics, based in Tianjin, which has also just begun clinical trials on a Covid-19 vaccine it developed with the military, according to Science Daily. Some 108 healthy volunteers in Wuhan aged between 18 and 60 have been injected with the vaccine, reports the state-run China Daily. Wang Junzhi, a fellow at the Chinese Academy of Engineering, said after receiving their injections, participants would spend 14 days in quarantine under observation. The volunteers will then receive a series of follow-up examinations within six months after the injection to see if their bodies have generated antibodies to the virus. The study’s slated to be completed by year-end.

China’s conducting simultaneously nine vaccine development projects, says drug and vaccine development expert, Wang YouTube/South China Morning Post
China conducting nine projects
Wang, who’s a drug and vaccine development expert, said China’s conducting simultaneously nine vaccine development projects. CanSino, which already has an Ebola vaccine on sale in China, has based its vaccine candidate on taking a Covid-19 genetic code snippet, enrobing it with a harmless virus, with the aim of creating Covid-19 antibodies. As well, Germany’s BioNTech has teamed up with China’s Fosun Pharma to develop a Covid-19 trial which is also seeking to create an antibody-spurring vaccine for Covid-19 based on bits of mRNA that it aims to move into clinical trials next month.
Another German biotech firm, CureVac, is also developing an mRNA vaccine. CureVac was recently at the heart of a political storm when the Trump Administration sought unsuccessfully to persuade the firm to shift its research work to the US, a move that spurred fears Washington was trying to poach the Covid-19 vaccine for use only in the United States.
US pharma heavyweight Johnson & Johnson, meanwhile, says it’s in the early days of developing a vaccine that would introduce patients to a deactivated version of the virus, triggering an immune response without causing infection. Human trials could begin by November. Fortune magazine, meanwhile, reports some researchers are working on “temporary vaccines” that might buy a couple months’ worth of protection while longer-lasting vaccines are developed.
The bottom line, though, is while there’s a lot out there looking promising for attacking Covid-19, there’s nothing immediately to halt the virus. Right now, the best thing governments can do is, the WHO says, is “test, test, test,” to break community transmission so patients can be isolated and treated early. And the best protection for individuals against the virus is to wash their hands and practice social distancing -- staying home, when outside remaining two metres away from others, avoiding crowds and parties and refraining from touching people. Even with the best research and will in the world, experts don’t believe a vaccine will be ready before 18 months -- and that timeframe would normally be considered very fast.










