|
 |
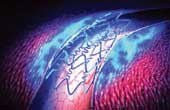
| A drug eluting stent (above) and a cross section of an artery showing how a stent is placed |
Two powerful enemies have got together to counter a common threat. Boston Scientific Corporation, a $25 billion corporation that develops products for minimally invasive surgical procedures, is working with its arch enemy, Cordis Corporation, part of the $200 billion behemoth, Johnson & Johnson. The agenda: studying a phenomenon called stent thrombosis.
Trouble suddenly erupted in September this year at the World Cardiology Congress in Barcelona when Donald Baim, chief scientific and medical officer of Boston Scientific, told a reporter that there were disturbing reports of late thrombosis after use of their Taxus stent. The incident created a huge buzz in medical circles.
So what is the fuss all about? And what is a stent?
A stent is a thin metal tube that is used to open a blocked coronary artery. Years of fat deposition resulting from unhealthy eating habits, stress, a lazy lifestyle, drinking and smoking cause the coronary arteries to get blocked by cholesterol deposits, a condition called atherosclerosis. The supply of oxygen and blood to the heart muscle is thus hampered, leading to a heart attack. The stent is deployed by the cardiologist through a wire passed via a groin artery. The tube keeps the blocked artery open, resuming blood supply.
However, the steel stent irritates the artery’s inner lining and muscle cells, creating a scar that clogs it. This is called re-stenosis. Since 2001, drugs that prevent this scar from forming were added to the stent. These are known as drug eluting stents (DES). In the Taxus stent made by Boston Scientific, the agent is an anti-cancer drug called paclitaxel, while in the Cypher stent made by Cordis, it is sirolimus. Many trials confirmed that the incidence of re-stenosis fell from 30 per cent in six months to around three per cent. This led to a sharp increase in the use of these costly (one costs over Rs 1 lakh) devices. Six million stents were used in just three years. The advent of DES not only led to a boom in invasive cardiology but also threatened to make bypass surgery superfluous in most cases.
Now after billions of dollars have been expended and countless lives saved and lost comes a series of trials, some showcased in the New England Journal of Medicine, showing that the DES are actually not much better than the simple, cheap, bare metal stents. Studies reveal that after the initial recovery period of 6-12 months, patients with DES have higher rates of heart attack and death than those with ordinary stents. This is because of late thrombosis (blood clotting) in the stent itself. To prevent such an eventuality, patients using stents are prescribed blood-thinning drugs like clopidogrel and aspirin for one or two years. Though some experts recommend a lifetime consumption, the cost and side effects are undesirable.
As the stent makers pour over the fine print to make sense of the trial statistics, the next generation of stents is already in place.
Rabin Chakraborty, head of the department of cardiology, Apollo Gleneagles Hospitals, Calcutta, says, “Second generation stents have a cobalt frame and are thinner and more flexible, thus allowing their use in smaller vessels, which we cannot access with stainless steel stents. One of these is XIENCE and the other is Endeavor, which are showing thrombosis rates of only 0.5 per cent.”
Scientists in New Zealand are now conducting human trials of the world’s first dissolvable third generation stent — the “bioabsorbable” stent that releases a drug called everolimus. The material used is like the one used in absorbable stitches. After two years of implantation, it disintegrates completely, having deployed the drug in the lining of the artery. This way, there is no “full metal jacket” in the artery that could act as a clot magnet.
How are the companies reacting to the adverse results? A. Vaidheesh, executive vice-president of Johnson & Johnson, India, downplays the impact of the studies. “The trials have included complicated cases of multiple vessel disease as well as patients with diabetes, well known as bad prognostic factors,” he says.
Medtronics Inc. is now conducting a huge trial, called the Protect Trial, for the first time in India (among other countries), comparing the results of their Endeavor stent with those of the Cypher by Cordis, specifically comparing the incidence of stent thrombosis between the two.
The latest treatment for stent blockage or re-stenosis is angioplasty using a paclitaxel-coated balloon, recently reported in the NEJM .
As things stand, the jury is still out on whether DES is an overrated product. Till such time, try not to get a heart attack!
Dr B. Ramana is a Calcutta-based advanced laparoscopic surgeon. He can be contacted at rambodoc@gmail.com