For the last 14 years, Amitava Sengupta has been studying blood - how blood cells are born, mature, die or turn into rogue cancer cells. Now, the cell biologist and biochemist at the Indian Institute of Chemical Biology (under Council of Scientific & Industrial Research) in Calcutta has inched closer to finding a switch that helps turn on a type of adult blood cancer.
The human body produces a trillion mature blood cells every day. Each one starts out as a haematopoietic (blood forming) stem cell (HSC) in the bone marrow and can mature into one of several subtypes of white and red blood cells. "It was in 2002 - as a research fellow at Saha Institute of Nuclear Physics in Calcutta - that I started delving into how normal blood cells develop," says Sengupta. During his postdoctoral study at Cincinnati Children's Hospital in the US, he figured out the finer details.
The knowledge of how a normal blood cell develops is crucial to understanding two key properties of stem cells - their ability to go through numerous cycles of cell division (or, self-renewal) and their capacity to differentiate into specialised blood cells. "When the self-renewal process gets impaired and the differentiation stops in HSC, blood cells turn cancerous or leukaemic," says Sengupta.
What Sengupta is trying to find is how the HSC changes to become a leukaemia cell. Are these switches hidden in our genes? In the DNA sequences?

Sengupta spent his initial years of research mapping the genetic profiles of blood cells that turn cancerous. Meanwhile, genetic techniques had helped other scientists pin genes that have the potential to cause cancer (oncogenes) and genes that protect a cell from turning cancerous (tumour suppressor genes). But these discoveries failed to use the knowledge of genes you carry to prevent or manage cancer.
"I learnt that many of these potentially fatal changes are scripted not just in genes," he says. "Specifically, there are changes that turn genes on or off without altering the sequences of DNA in the gene. These are called epigenetic changes."
In simplified terms, epigenetics is the study of biological mechanisms that will switch genes on and off. What you eat, where you live, who you interact with, when you sleep, how you exercise, even ageing - all of these can eventually cause chemical modifications around the genes that will turn those genes on or off. Additionally, in certain diseases such as cancer, various genes will be switched into the opposite state, away from the normal or healthy state. However, there is a silver lining: epigenetic imprints are reversible. If we could map every single cause and effect of the different combinations, and if we could reverse the gene's state to keep the good while eliminating the bad then we could theoretically cure cancer, slow ageing, stop obesity, and do so much more.
Early breakthroughs in epigenetics were elaborately explained by Siddhartha Mukherjee in his book The Gene: An Intimate History. The book features his mother and aunt, identical twins who have distinct personalities. He wrote that identical twins differ because: "Chance events - injuries, infections, infatuations; the haunting trill of that particular nocturne - impinge on one twin and not on the other. Genes are turned on and off in response to these events, as epigenetic marks are gradually layered above genes, etching the genome with its own scars, calluses, and freckles."
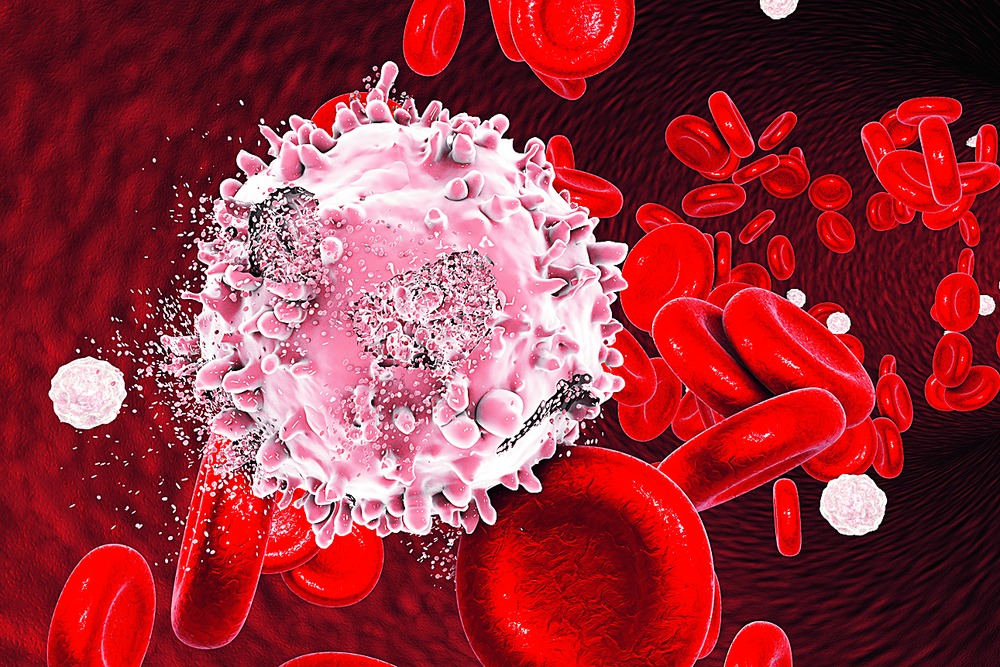
To understand how epigenetics works in cancer cells, Sengupta chose adult acute myeloid leukaemia (AML). AML accounts for around 80 per cent acute leukaemia in adults. Abnormal immature white blood cells fill the bone marrow and spill into the bloodstream. Production of normal blood cells is affected, causing anaemia, bleeding problems and infections.
Sengupta's clinical collaborator is Dr Debasis Banerjee, a senior haemato-oncologist at Park Clinic in Calcutta. "Patients [with AML] usually have a short history (one to eight weeks) of complaints such as fatigue, lack of energy, profuse sweating, bleeding from the gums or bruising in the skin. Anaemia and low platelet count are usual," he says. According to Dr Banerjee, most cases of AML occur as a consequence of somatic mutation - genetic alteration in a cell that can be passed on in the course of cell division - in HSCs. "In our study we found it usually hits those over 48," says Sengupta. Such mutations are frequently caused by environmental factors, such as exposure to ultraviolet radiation or to certain chemicals such as benzene - used in polymers, lubricants and pesticides.
"We have been trying to understand how the HSCs turn cancerous," Sengupta says, "And how things beyond genes have a role in causing cancer." Their focus is on a material called chromatin that packages a large amount of DNA inside a cell and keeps it organised. There are certain proteins called histones that are used as a scaffold for chromatin. "We found that there are certain regulators - Switch/Sucrose Non-Fermentable (SWI/SNF) - in chromatin that play a deterministic role in the genesis of tumour," he says. The loss of this regulator leads to formation of a complex assembly of protein that turns on the cancer switch. A part of the finding, co-authored by a team of researchers led by Sengupta, will be presented next month at Keystone Symposia on Haematopoiesis in Alberta, Canada.
He believes targeting the epigenetic switch in a selective manner can pave the way to a novel chemical therapy in future. The chemotherapy drugs currently used for treatment of AML (such as, daunorubicin and cytarabine) were developed way back in the 1960s.
"It seems the epigenetic signature is closely related to ageing in our body," Sengupta says. Perhaps, this is the reason why the incidence rate of AML increases with age. Finding out the precise cancer trigger can also help doctors diagnose the disease at an earlier stage. Sengupta's lab is also investigating the epigenetic cues in myelodysplastic syndrome (MDS), a kind of early stage leukaemia associated with ageing. Apparently, before full-blown AML sets in, the disorder goes through lower risk MDS stages. "Unfortunately, most diagnoses happen at a mature stage when no potential therapy is effective," he adds.
But that's a long way to go - it can take years, or even a few decades, before the researchers lay their hands on that epigenetic switch. And that elusive switch can be a huge breakthrough for not just AML, but a wide variety of cancers.